
News OEM
Hepatitis: Still a Global Health Issue
April 19, 2021 - Spain

One of the major causes of acute and chronic liver diseases is infection by the Hepatitis B and C viruses (HBV and HCV respectively). Both are blood-borne viruses and therefore their transmission occurs due to exposure to infected blood or bodily fluids containing blood. It is estimated that HBV and HCV infections cause 1.4 million deaths annually1.
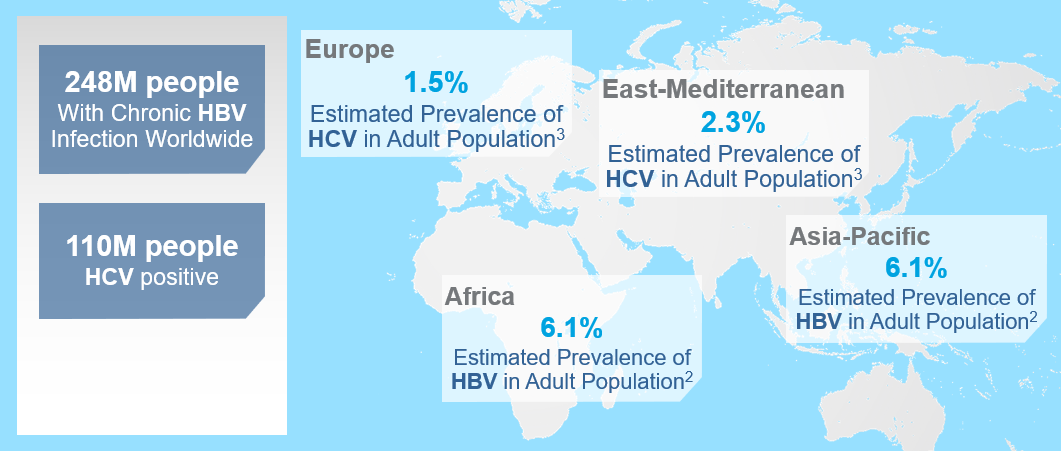
It is estimated that around 248 million people worldwide are living with chronic HBV infection while 110 million people worldwide are HCV positive. The highest HBV prevalence is in the Asia-Pacific and Africa regions with an estimated prevalence in the adult population of 6.2% and 6.1%, respectively2. For HCV infection, the prevalence is highest in the Eastern Mediterranean and European regions with an estimated incidence in the adult population of 2.3% and 1.5%, respectively3.
Although HBV vaccination campaigns and improved treatments for HBV and HCV have played a key role in controlling the spread of these viruses, these infections are still a global healthcare issue.
Hepatitis is a global health issue that is leading to an increase in the demand for Hepatitis immunoassays. Are you ready for that?
If you are expecting an increase in demand for your HBV and/or HCV assays, or you envision a new generation of these immunoassays in your portfolio, we can help you with Biokit Hepatitis B and C Biomaterials. Our Biomaterials are playing a key role in the commercial and clinical success of various IVD assays in the chemiluminescence format.
When used in commercial chemiluminescence IVD assays, Biokit Hepatitis B and C Biomaterials have demonstrated the following performance:
|
|
Commercial IVD Assay |
Sensitivity |
Specificity |
|
Hepatitis B |
Hepatitis B surface Antigen (HBsAg) |
100% n=537* |
99.06% n=5,181† |
|
Hepatitis B core Antigen (HBcAg) |
100% n=403* and n=203* |
99.9% n=5,097† |
|
|
Hepatitis B surface Antigen Antibodies (anti-HBs) |
99.0% n=349‡ |
100% n=349‡ |
|
|
Hepatitis C |
Hepatitis C IgG Antibody (anti-HCV) |
100% n=704* |
99.7% n=5,022† |
*Positive samples from various sources. †Samples from blood donors. ‡Sample population.
Biokit Hepatitis biomaterials portfolio consists of the following products:
|
|
Biomaterial |
Description |
PN |
|
Hepatitis B |
Antigens |
Hepatitis B Surface Antigen (ad subtype) |
3000-5201 |
|
Hepatitis B Surface Antigen (ay subtype) |
3000-5199 |
||
|
Recombinant HBs Antigen (ad subtype) |
3000-5269 |
||
|
Recombinant HBc Antigen |
3000-5100 |
||
|
Antibodies |
Anti-HBs Goat IgG pAb |
3000-5501 |
|
|
Anti-HBs Guinea Pig IgG pAb |
3000-5264 |
||
|
Anti-HBs mAb IgG |
3000-5012 |
||
|
Hepatitis C |
Antigens |
Recombinant HCV Capsid Polypeptide |
3000-5103 |
|
Recombinant HCV NS3 Polypeptide |
3000-5104 |
Sources: 1WHO (World Health Organization). WHO Guidelines on Hepatitis B and C Testing [Internet]. Geneva: WHO; 2017 Feb. 204 p. [cited 2018 May 23]. Available from: http://www.who.int/hepatitis/publications/guidelines-hepatitis-c-b-testing/en/; 2World Health Organization (WHO). Hepatitis B [Internet]. Geneva: WHO; 2019 Jul 18. (Fact sheets; ). [cited 2020 Mar 1]. Available from: https://www.who.int/news-room/fact-sheets/detail/hepatitis-b; 3World Health Organization (WHO). Hepatitis B [Internet]. Geneva: WHO; 2019 Jul 18. (Fact sheets; ). [cited 2020 Mar 1]. Available from: https://www.who.int/news-room/fact-sheets/detail/hepatitis-b
NEWS
Latest News
Please contact us directly via telephone or with the following form.
Tel. +34 93 860 90 00

