
News OEM
Overcoming Challenges Together II
July 2, 2020 - Spain

Biokit’s Immunoassay manufacturing capabilities can be fitted to our partners’ needs
Today and in the coming years, due to the changing market, major IVD players will have to meet the increased demand for IVD products while remaining innovative, reliable, and focused on quality.
Our experience in immunoassay development and manufacturing helps us to understand the challenges you might face as an immunoassay manufacturer when you have to adapt to an increase in demand, either for the final immunoassay or for the key IVD biomaterials, such as antigens and antibodies.
Our routine manufacturing currently includes several different monoclonal antibodies (mAb) that mainly go on to be used as the key IVD biomaterial in different commercial immunoassays for a variety of medical conditions.
In Biokit, we work alongside our partners to help them meet their operational and quality needs.
Our approach
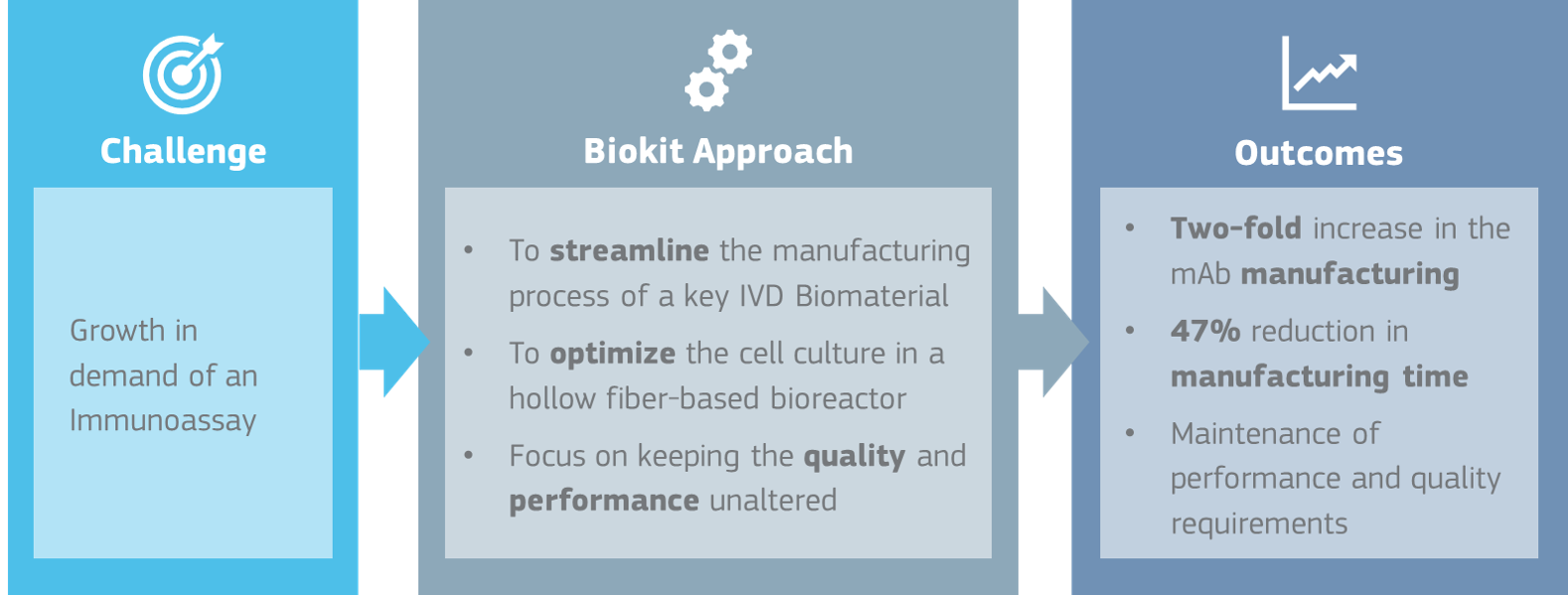
Recently, one of our partners, experienced an increase in demand from their customers for a specific immunoassay. In order to support our partner, our operations team has worked to streamline the manufacturing of one of the monoclonal antibodies being used in this commercial immunoassay, so we can supply this key IVD biomaterial according to our partner’s operational needs.
To pursue this objective, our Cell Culture Department evaluated which would be the optimal cell culture system while keeping the same expression system and mAb specifications. This evaluation kept sight of the fact that the mAb is being used in a commercial immunoassay, meaning the performance and quality requirements should remain the same. The selected approach was to move from a membrane-based cell culture to a hollow fiber-based bioreactor once the cell culture had been optimized to this type of bioreactor.
Outcomes
As a result of our approach, after optimizing the cell culture and switching to the hollow fiber-based bioreactor we were able to increase mAb manufacturing two-fold, with a time reduction of 47%, while also meeting our partner’s operational needs and immunoassay performance and quality requirements.
NEWS
Latest News
Please contact us directly via telephone or with the following form.
Tel. +34 93 860 90 00

