
News OEM
Syphilis strikes back again
September 25, 2019

Syphilis is a sexually transmitted disease (STD) caused by the pathogen Treponema Pallidum. It can affect different organ systems, mainly in the heart, circulatory system and nervous system. Syphilis can spread from an infected mother to her unborn baby, leading to potential pregnancy complications.
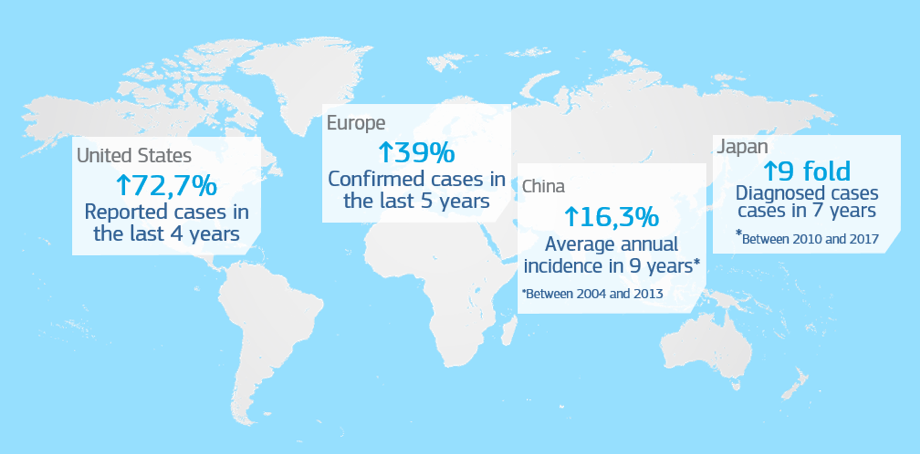
In recent years, there has been an increase in the incidence rate of syphilis among the population. In the US, the rate of both primary and secondary syphilis has shown an increase1 of 72.7% when comparing the cases reported between 2013 and 2017.
In Europe, the rate of both primary and secondary syphilis has shown an increase2 of 39% when comparing the cases reported between 2012 and 2017.
The scenario in Asia is not optimistic either: in Japan, the rate of both primary and secondary syphilis has shown a dramatic increase3, from 621 reported cases in 2010 to 5770 cases in 2017 (9 fold increase), and in the case of China, the average annual increase in the incidence of syphilis between 2004 and 2013 was 16.3%4
[1]Centers for Disease Control and Prevention. Syphilis Surveillance supplement 2013-2017. Atlanta: U.S. Department of Health and Human Services.
[2]European Centre for Disease Prevention and Control. Syphilis. In: ECDC. Annual epidemiological report for 2016. Stockholm: ECDC; 2018.
[4]Wong et al. Distribution of reported syphilis cases in South China: spatiotemporal analysis. Nature Scientific Reports.2018
This increase in the incidence will lead to an increase in the demand for Syphilis immunoassays. Are you ready for that?
If you are expecting an increase of demand of your Syphilis assay or you envision a new generation of Syphilis immunoassays for your portfolio, we can help you with Biokit Syphilis Biomaterials. Biokit Syphilis Biomaterials are playing a key role in the commercial and clinical success of different IVD assays, in either ELISA or Chemiluminescence format.
When used in a commercial ELISA IVD assay, Biokit Syphilis Biomaterials have demonstrated clinical proficiency by obtaining a global relative sensitivity of 99.4 % with a panel of characterized positive samples from various sources (n=159), and 100% when the ELISA assay was evaluated with a commercial panel (n=25). The specificity of the ELISA commercial assay that uses Biokit Syphilis Biomaterials is 99.8% from Blood donors (2883) and 100% when the ELISA assay was evaluated with a commercial panel (n=25 ). The specificity of the ELISA commercial assay that uses Biokit Syphilis Biomaterials is 99.8% from Blood donors (2883) and 100% from samples of hospitalized patients.
Biokit Syphilis Biomaterials have been used also in a commercial Chemiluminescence IVD assay with excellent clinical performance, obtaining a global relative sensitivity of 100% (n=496) and a relative specificity of 100% (n=496), with an overall agreement of 100% with the predicate device (n=496).
Biokit Syphilis Biomaterials consist of the following antigens:
|
Description |
PN |
|
Treponema pallidum recombinant p15 antigen |
3000-5289 |
|
Treponema pallidum recombinant p17 antigen |
3000-5280 |
|
Treponema pallidum recombinant p47 antigen |
3000-5284 |

NEWS
Latest News
Please contact us directly via telephone or with the following form.
Tel. +34 93 860 90 00

